TIME TO CONSIDER TREATMENT WITH SOHONOS
The FIRST AND ONLY treatment to reduce new heterotopic ossification (HO) in fibrodysplasia ossificans progressiva (FOP)1
This illustration was co-created with people living with FOP.
How SOHONOS inhibits HO formation
SOHONOS inhibits chondrogenesis, which is directly responsible for HO formation.1
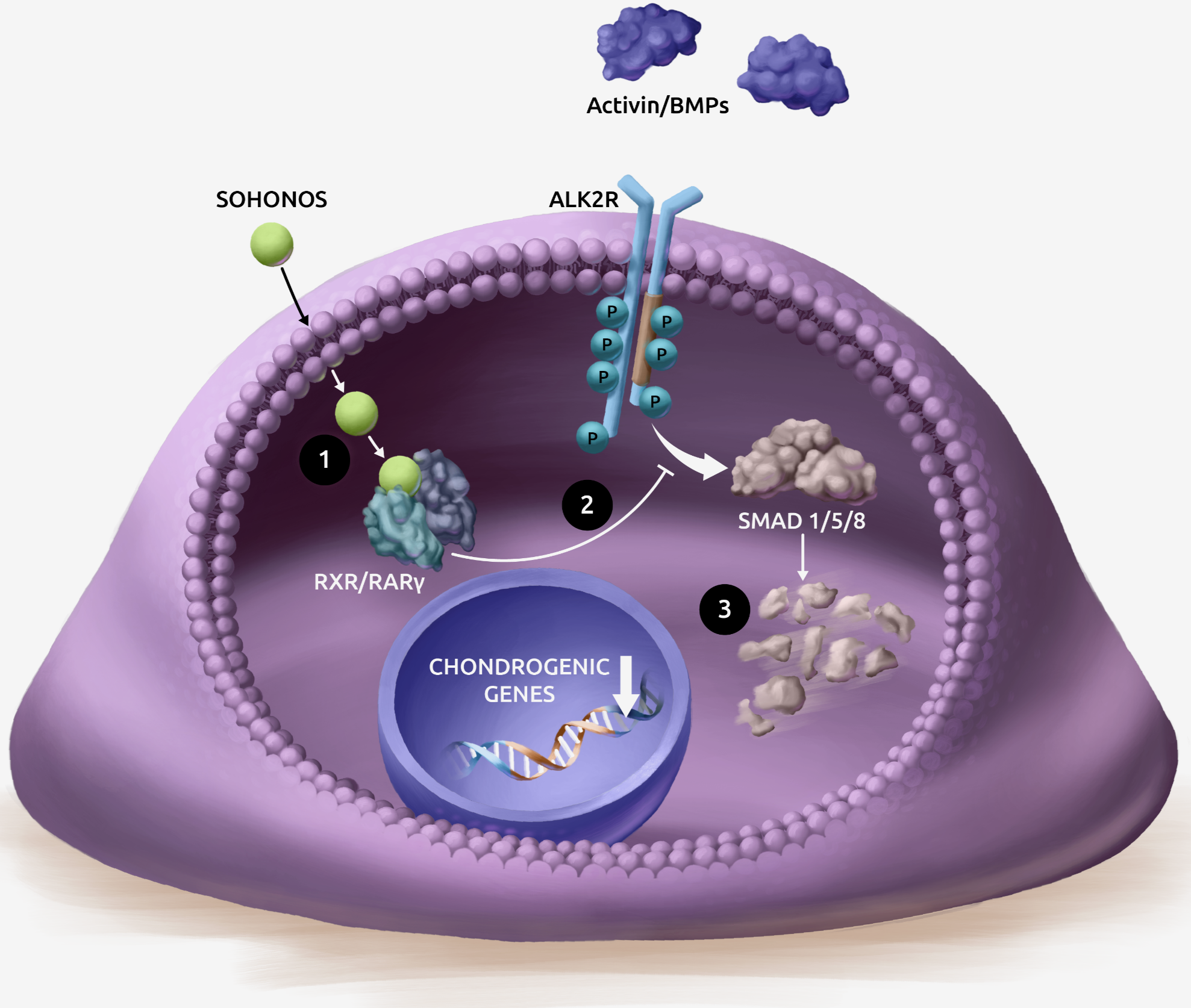
How SOHONOS inhibits HO formation
SOHONOS inhibits chondrogenesis, which is directly responsible for HO formation.1
- SOHONOS is an orally bioavailable retinoic acid receptor agonist, with particular selectivity at the gamma subtype of RAR (RARγ)2-4
- SOHONOS binding to RARγ activates retinoid signaling, inhibiting both aberrant Activin A-receptor and BMP-SMAD 1/5/8 pathway signaling in FOP4,5
- This reduces ALK2/SMAD-dependent chondrogenesis and osteocyte differentiation resulting in reduced new endochondral bone formation1

SOHONOS efficacy and safety were evaluated in a Phase 3 clinical trial6
MOVE was an open-label, single-arm, multicenter, clinical trial evaluating SOHONOS for patients with FOP6
The MOVE trial began in November 2017 and was completed in September 2022. Adult and pediatric patients (N=97) with FOP who had a confirmed ACVR1R206H mutation and were ≥4 years of age were eligible to participate. Only patients with a baseline and at least one post-baseline HO volume measurement were included in the efficacy analysis.1,6,7
Data from a natural history study (NHS), PVO-1A-001, was used as an external control for the MOVE trial. The NHS was a 36-month, noninterventional, longitudinal study of untreated patients with FOP (N=101) and an ACVR1R206H mutation.1,8
MOVE was a 48-month clinical trial with 2 parts6

There was a partial clinical hold (initiated December 2019) applied to the pediatric population (patients <14 years of age) actively participating in MOVE in all clinical trial sites globally.
Primary endpoint: Annualized change in new HO volume versus untreated natural history participants assessed by low-dose WBCT (excluding the head).6
Analyzed using a Bayesian compound Poisson model with square-root transformation. The difference in visit schedules between MOVE and the NHS (WBCT scans every 6 and 12 months, respectively), with the square-root transformation, introduced a bias, particularly in the Bayesian analysis, and therefore a non-transformed mean annualized volume of new HO was also conducted.6
WBCT=Whole body computed tomography.
MOVE was the largest interventional clinical trial for patients with FOP
The demographics and baseline disease characteristics, including flare-up history, were sufficiently similar between the Phase 3 clinical trial and the NHS to support comparison of the results between the studies.6
While HO data were adjusted for covariates due to differences in baseline characteristics between MOVE and the NHS, significant effects of unknown confounding factors cannot be ruled out given the lack of randomization.6

*There were more male than female subjects in both the SOHONOS (MOVE) and untreated groups (NHS).
Dosing in MOVE was adjusted based on patient age, weight, and the presence of a flare-up6
Adult/pediatric patients received SOHONOS 5 mg daily and were escalated during flare-ups (at least one symptom [e.g., pain, swelling, redness] consistent with a previous flare-up or a substantial high-risk traumatic event likely to lead to a flare-up).6
- Flare-up dosing was 20 mg once daily for 4 weeks followed by 10 mg once daily for 8 weeks
- The 12-week treatment was restarted if the patient had another flare-up or substantial high-risk traumatic event, and/or extended in 4-week increments for persistent symptoms
Chronic and flare-up dosing was adjusted according to body weight in skeletally immature children (patients <18 years old with <90% skeletal maturity [bone age <12 years in females and <14 years in males]).6
SOHONOS was proven to reduce new heterotopic ossification (HO)1,6
SOHONOS demonstrated a 54% reduction in new HO volume compared to untreated patients from an NHS1,6
The treatment effect was ~10.9 cm3/year with 95% confidence interval (-21.2 cm3/year, -0.6 cm3/year)1
The annualized new HO volume observed in SOHONOS-treated patients was approximately half of that observed in untreated people with FOP from the NHS1
- These results represent a post-hoc analysis of a Phase 3 clinical trial using a linear mixed effect model. The pre-specified Bayesian statistical analysis included a square root transformation that masked the effect of treatment with SOHONOS6

Reducing the volume of new HO is key to slowing disease progression9-12
SOHONOS safety profile1

Embryo-fetal toxicity1
Advise patients that SOHONOS can cause fetal harm and is contraindicated during pregnancy.
Obtain a negative serum pregnancy test within 1 week prior to initiating SOHONOS. Verify that your patient is not pregnant periodically, as needed, over the course of treatment and 1 month after discontinuation unless they are not at risk of pregnancy.
Advise females of reproductive potential to:
- Avoid pregnancy while taking SOHONOS and for at least one month following discontinuation of therapy
- Use at least one highly effective method of contraception (i.e., IUD) or two effective methods (i.e., combined hormonal contraception in combination with another method of contraception such as a barrier method) during treatment with SOHONOS
- Immediately stop taking SOHONOS if they become pregnant while taking this drug and rapidly consult their healthcare provider if there is a risk of pregnancy or if they might be pregnant
Because of the potential for serious adverse reactions from SOHONOS in a breastfed child, advise patients not to breastfeed during treatment with SOHONOS, and for at least 1 month after their last dose.
Administration of SOHONOS to a male patient is considered unlikely to affect development of an embryo or fetus carried by a pregnant female sexual partner exposed to SOHONOS via the patient’s semen.

Premature epiphyseal closure in growing pediatric patients1
Inform patients that SOHONOS has been shown to cause premature epiphyseal closure in growing pediatric patients with FOP.
- Prior to starting treatment with SOHONOS, all growing children should undergo baseline clinical and radiological assessments.* Continued monitoring is recommended every 6-12 months until patients reach skeletal maturity (e.g., epiphyseal closure) or final adult height
*Including but not limited to assessment of skeletal maturity via hand/wrist and knee x-rays, standard growth curves and pubertal staging.
In clinical studies, premature epiphyseal closure was identified as an irreversible serious risk associated with SOHONOS treatment.1
27% of patients <18 years of age experienced premature epiphyseal closure, which was more common in younger patients:
- 31% in patients aged ≥8/10 to <14 years
- No patients ≥14 years
- Many of the affected patients exhibited slowing of growth in height

Radiological vertebral fractures1
Inform patients that SOHONOS has resulted in decreased vertebral bone mineral content, bone density and bone strength as well as an increased risk of radiologically observed vertebral fractures and that periodic radiological assessment of the spine is recommended.

Mucocutaneous adverse effects1
Mucocutaneous effects were the most commonly reported adverse events across all doses during clinical studies with SOHONOS (98%).
Mucocutaneous AEs leading to dose reductions were more common during SOHONOS 20/10 mg flare-up treatment (37%) than during chronic treatment (4%)
Adverse mucocutaneous reactions with >10% frequency reported in patients treated with SOHONOS (females ≥8 and males ≥10)
Adverse mucocutaneous reactions reported >10% frequency
aIncludes lip dry, chapped lips, cheilitis. bIncludes pruritus, pruritus generalized, and rash pruritic. cIncludes rash, rash generalized, rash maculo-papular. dIncludes erythema, generalized erythema, flushing, rash erythematous.
Adverse mucocutaneous reactions with >10% frequency reported in patients treated with SOHONOS (females ≥8 and males ≥10)

Adverse mucocutaneous reactions reported >10% frequency
aIncludes lip dry, chapped lips, cheilitis. bIncludes pruritus, pruritus generalized, and rash pruritic. cIncludes rash, rash generalized, rash maculo-papular. dIncludes erythema, generalized erythema, flushing, rash erythematous.
Proactively manage mucocutaneous effects1
Advise patients that they may experience mucocutaneous effects. Prophylactic measures to minimize risk and/or treat the mucocutaneous effects are recommended, such as:
- Skin emollients
- Sunscreen
- Lip moisturizers
- Artificial tears
- Other helpful treatments
- Skin emollients
- Artificial tears
- Sunscreen
- Other helpful treatments
- Lip moisturizers

Get the
Mucocutaneous Brochure
Learn more about mucocutaneous effects and how they can be managed

Psychiatric disorders and night blindness1
New or worsening psychiatric events were reported with SOHONOS. Monitor for development of new or worsening psychiatric symptoms including depression, anxiety, mood alterations and suicidal thoughts and behaviors. Night blindness has been associated with systemic retinoids, including SOHONOS. Advise patients to be cautious when driving or operating any vehicle at night and to seek medical attention in the event of vision impairment.
Clinical trial experience
Summary of adverse events reported at >10% frequency in FOP Patients ≥8 (female)/≥10 (male) years of age in clinical trials1

Doses were reduced according to body weight in patients who were less than 90% skeletally mature.
aIncludes lip dry, chapped lips, cheilitis. bIncludes pruritus, pruritus generalized, and rash pruritic. cIncludes rash, rash generalized, rash maculo-papular. dIncludes erythema, generalized erythema, flushing, rash erythematous. eIncludes headache and migraine. fIncludes back pain, flank pain, sciatica. gIncludes myalgia, musculoskeletal discomfort. hIncludes drug eruption, hypersensitivity, pruritus allergic, drug hypersensitivity. iIncludes peripheral swelling, edema peripheral. jIncludes fatigue, lethargy, asthenia, malaise.
IMPORTANT SAFETY INFORMATION
WARNING: EMBRYO-FETAL TOXICITY and PREMATURE EPIPHYSEAL CLOSURE IN GROWING PEDIATRIC PATIENTS
- SOHONOS is contraindicated in pregnancy. SOHONOS may cause fetal harm. Because of the risk of teratogenicity and to minimize fetal exposure, SOHONOS is to be administered only if conditions for pregnancy prevention are met.
- Premature epiphyseal closure occurs in growing pediatric patients treated with SOHONOS, close monitoring is recommended.
Contraindications
SOHONOS is contraindicated in patients during pregnancy, or with a history of allergy or hypersensitivity to retinoids, or to any component of SOHONOS. Anaphylaxis and other allergic reactions have occurred with other retinoids.
Warnings and Precautions
- Embryo-Fetal Toxicity: SOHONOS can cause fetal harm and is contraindicated during pregnancy. SOHONOS is a retinoid which is associated with birth defects in humans. Advise females of reproductive potential to use an effective method of contraception at least 1 month prior to treatment, during SOHONOS treatment and for 1 month after the last dose. If a pregnancy occurs during treatment, discontinue treatment immediately and refer the patient to an obstetrician/gynecologist experienced in reproductive toxicity. Inform patients not to donate blood during SOHONOS treatment and for 1 week following discontinuation.
- Premature Epiphyseal Closure in Growing Pediatric Patients: SOHONOS can cause irreversible premature epiphyseal closure and potential adverse effects on growth. In clinical studies, premature epiphyseal closure occurred with SOHONOS treatment in growing pediatric patients with FOP. Monitoring of linear growth is recommended in growing pediatric patients. Prior to starting treatment with SOHONOS, all growing pediatric patients should undergo baseline assessment of skeletal maturity and continued monitoring until patients reach skeletal maturity or final adult height. If a patient exhibits signs of premature epiphyseal closure or adverse effects on growth based on clinical or radiologic evaluations, further evaluation may be required, including an assessment of the benefits and risks of continued treatment, or temporary or permanent discontinuation of SOHONOS until the patient achieves epiphyseal closure and skeletal maturity.
- Mucocutaneous Adverse Reactions: Dry skin, lip dry, pruritus, rash, alopecia, erythema, skin exfoliation (skin peeling), and dry eye occurred in 98% of patients treated with SOHONOS. SOHONOS may contribute to an increased risk of skin and soft tissue infections, particularly paronychia and decubitus ulcer, due to a decreased skin barrier from adverse reactions such as dry and peeling skin. Some of these adverse reactions led to dose reductions which occurred more frequently during flare-up dosing suggesting a dose response relationship. Prophylactic measures to minimize risk and/or treat the mucocutaneous adverse reactions are recommended (e.g., skin emollients, sunscreen, lip moisturizers, or artificial tears). Some may require dose reduction or discontinuation. Photosensitivity reactions (e.g., burning, erythema, blistering) involving areas exposed to the sun have been associated with the use of retinoids and may occur with SOHONOS. Precautionary measures for phototoxicity are recommended (use of sunscreens, protective clothing, and use of sunglasses).
- Metabolic Bone Disorders: Retinoids are associated with bone toxicity, including reductions in bone mass and spontaneous reports of osteoporosis and fracture. In FOP clinical studies, SOHONOS resulted in decreased vertebral bone mineral content and bone density, and an increased risk of radiologically observed vertebral fractures in treated patients compared to untreated patients. Periodic radiological assessment of the spine is recommended. Retinoids have been associated with hyperostotic changes (bone spurs) and calcification of tendons or ligaments may occur with SOHONOS.
- Psychiatric Disorders: New or worsening psychiatric events were reported with SOHONOS including depression, anxiety, mood alterations, and suicidal thoughts and behaviors. There is a relatively high background prevalence of psychiatric disorders in untreated patients with FOP. Monitor for development of new or worsening psychiatric symptoms during treatment with SOHONOS. Individuals with a history of psychiatric illness may be more susceptible to these adverse effects. Patients and/or caregivers should contact their healthcare provider if new or worsening psychiatric symptoms develop during treatment with SOHONOS.
- Night Blindness: This may be dose-dependent, making driving a vehicle at night potentially hazardous during treatment. Advise patients to be cautious when driving or operating any vehicle at night and seek medical attention in the event of vision impairment.
Adverse Reactions
The most common adverse reactions (≥ 10%) are dry skin, lip dry, arthralgia, pruritus, pain in extremity, rash, alopecia, erythema, headache, back pain, skin exfoliation (skin peeling), nausea, musculoskeletal pain, myalgia, dry eye, hypersensitivity, peripheral edema, and fatigue.
Drug Interactions
- CYP3A4 inhibitors may increase SOHONOS exposure. Avoid concomitant use of strong or moderate CYP3A4 inhibitors, as well as grapefruit, pomelo or juices containing these fruits.
- CYP3A4 inducers may decrease SOHONOS exposure. Avoid concomitant use of strong or moderate CYP3A4 inducers.
- The use of both vitamin A and SOHONOS at the same time may lead to additive effects. Concomitant administration of vitamin A in doses higher than the recommended daily allowance and/or other oral retinoids must be avoided due to risk of hypervitaminosis A.
- Systemic retinoid use has been associated with cases of benign intracranial hypertension (pseudotumor cerebri), some of which involved the concomitant use of tetracyclines. Avoid coadministration of SOHONOS with tetracycline derivatives.
Use in Specific Populations
- Pregnancy: SOHONOS is contraindicated during pregnancy. Obtain a negative serum pregnancy test within 1 week prior to SOHONOS therapy and periodically, as needed, over the course of treatment with SOHONOS and 1 month after treatment discontinuation unless patient is not at risk of pregnancy. If pregnancy occurs during treatment with SOHONOS, stop treatment immediately and refer the patient to an obstetrician/gynecologist or other specialist experienced in reproductive toxicity for evaluation and advice.
- Lactation: Advise females that breastfeeding is not recommended during treatment with SOHONOS, and for at least 1 month after the last dose.
- Females and Males of Reproductive Potential: Advise females of reproductive potential to use effective contraception at least 1 month prior to and during treatment, and for 1 month after the last dose unless continuous abstinence is chosen.
- Pediatric Use: All growing pediatric patients should undergo baseline assessment of growth and skeletal maturity before starting treatment and continued clinical and radiographic monitoring every 6-12 months until patients reach skeletal maturity or final adult height.
- Renal or Hepatic Impairment: Use of SOHONOS in patients with severe renal impairment, or with moderate or severe hepatic impairment is not recommended.
INDICATION
SOHONOS™ is indicated for the reduction in volume of new heterotopic ossification in adults and pediatric patients aged 8 years and older for females and 10 years and older for males with fibrodysplasia ossificans progressiva (FOP).
Please see full Prescribing Information, including BOXED WARNING.
References: 1. SOHONOS Full Prescribing Information. Cambridge, MA: Ipsen Biopharmaceuticals, Inc; August 2023. 2. Sanchez‑Duffhues G, de Gorter DJJ, ten Dijke P. Towards a cure for fibrodysplasia ossificans progressiva. Ann Transl Med. 2016;4:S28. doi: 10.21037/atm.2016.10.62. 3. Anwar S, Yokota T. Navigating the complex landscapes of fibrodysplasia ossificans progressiva: from current paradigms to therapeutic frontiers. Genes. 2023;14:2162. doi.org/10/3390/genes14122162. 4. Pignolo RJ, Pacifici M. Retinoid agonists in the targeting of heterotopic ossification. Cells. 2021;10:3245. https://doi.org/10.3390/cells10113245. 5. Shimono K, Tung W, Macolino C. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor γ agonists. Nat Med. 2011;17(4):454‑460. 6. Pignolo RJ, Hsiao EC, Al Mukaddam M, et al. Reduction of new heterotopic ossification (HO) in the open-label, phase 3 MOVE trial or palovarotene for fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2023;38(3):381-394. 7. Ipsen (Clementia Pharmaceuticals Inc.) An efficacy and safety study of palovarotene for the treatment of fibrodysplasia ossificans progressiva. (MOVE). https://clinicaltrials.gov/study/NCT03312634. Clinicaltrials.gov identifier: NCT03312634. Updated November 29, 2023. Accessed February 26, 2024. 8. Ipsen (Clementia Pharmaceuticals Inc.) A natural history study of fibrodysplasia ossificans progressiva (FOP). https://www.clinicaltrials.gov/study/NCT02322255?term=NCT02322255&rank=1. Clinicaltrials.gov identifier: NCT02322255. Updated June 06, 2020. Accessed February 26, 2024. 9. Di Rocco M, Baujat G, Bertamino M, et al. International physician survey on management of FOP: a modified Delphi study. Orphanet J Rare Dis. 2017;12(1):110. 10. Pignolo RJ, Bedford- Gay C, Liljesthröm M, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res. 2016;31(3):650-656. 11. Pignolo RJ, Baujat G, Brown MA, et al. Natural history of fibrodysplasia ossificans progressiva: cross-sectional analysis of annotated baseline phenotypes. Orphanet J Rare Dis. 2019;14(1):98. 12. Baujat G, Choquet R, Bouée S, et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: an estimate based on a record linkage of two national databases. Orphanet J Rare Dis. 2017;12(1):123.
