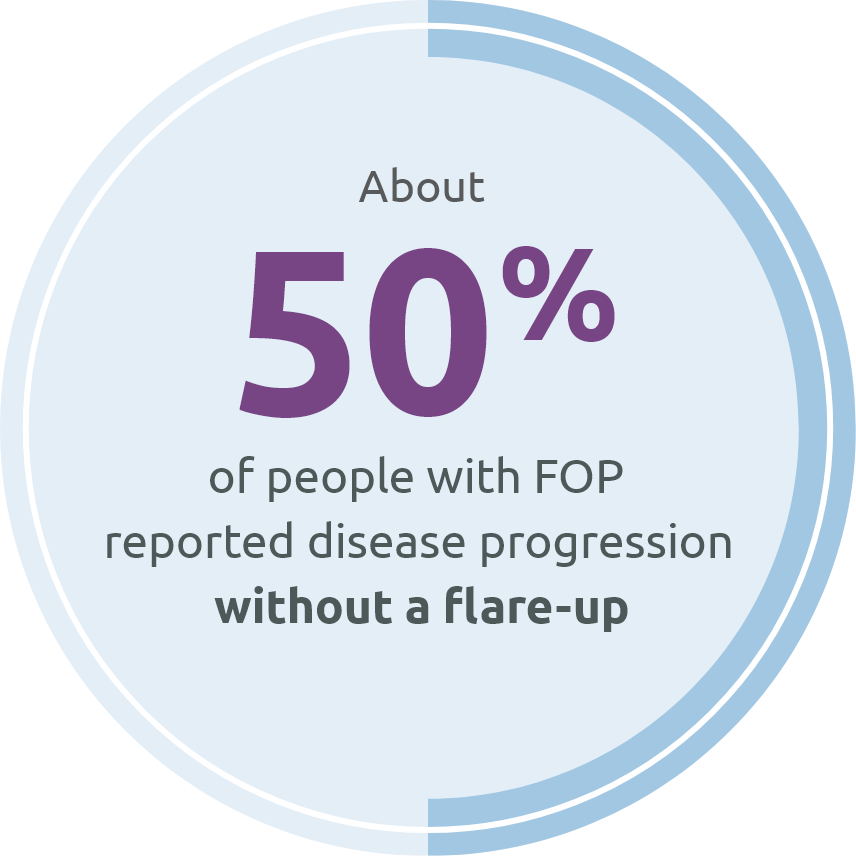
In a global survey of 500 people with FOP:

Once HO forms, it is irreversible.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SOHONOS?
SOHONOS can cause birth defects (deformed babies) if taken during pregnancy. Females who are pregnant or who plan to become pregnant must not take SOHONOS.
- Your healthcare provider will ask you to take a pregnancy test 1 week before starting treatment with SOHONOS, periodically during treatment, and 1 month after you stop treatment.
- You must use effective birth control (contraception) starting at least 1 month before starting treatment with SOHONOS, during treatment, and for 1 month after the last dose. Talk to your healthcare provider about birth control methods that may be right for you.
- If you become pregnant or think you may be pregnant during treatment with SOHONOS, stop taking SOHONOS and call your healthcare provider right away.
Because SOHONOS can cause birth defects, SOHONOS is only for people who can understand and agree to carry out all instructions for pregnancy prevention.
SOHONOS can cause bone growth changes. Children may stop growing while taking SOHONOS. Bone growth changes such as permanent early closure of the growth plate in growing children have happened with SOHONOS. Your healthcare provider will closely monitor your child’s bone growth and height during treatment with SOHONOS.
Who should not take SOHONOS?
Do not take SOHONOS if you are pregnant, or allergic to medicines known as retinoids or any of the ingredients in SOHONOS.
What should I tell my healthcare provider before taking SOHONOS?
Before taking SOHONOS, tell your healthcare provider about all your medical conditions, including:
- have bone loss (osteoporosis), weak bones or any other bone problems
- have or had mental health problems
- have or have had kidney problems
- have or have had liver problems
- are breastfeeding or plan to breastfeed. It is not known if SOHONOS passes into your breastmilk. Breastfeeding is not recommended during treatment with SOHONOS and for at least 1 month after the last dose of SOHONOS. Talk to your healthcare provider about the best way to feed your baby if you take SOHONOS.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. SOHONOS and certain other medicines can interact with each other, sometimes causing serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist when you get a new medicine.
What should I avoid while taking SOHONOS?
- Do not get pregnant while taking SOHONOS.
- Avoid excessive exposure to sunlight and ultraviolet lights (tanning machines). SOHONOS may make your skin more sensitive to the exposure and you may burn more easily. Apply sunscreen and wear protective clothing and sunglasses when in sunlight.
- Avoid driving at night until you know if SOHONOS has affected your vision. SOHONOS may decrease your ability to see in the dark.
- Do not donate blood while taking SOHONOS and for 1 week after stopping SOHONOS.
What are the possible side effects of SOHONOS?
SOHONOS can cause serious side effects, including:
- skin-related events such as dry skin, lip and eye, hair loss, itching, redness, rash, and skin peeling. You may be at increased risk of developing skin and soft tissue infections while taking SOHONOS. If you develop these symptoms, your healthcare provider may tell you to use moisturizer, sunscreen, or artificial tears.
- bone mineral density problems (bone thinning) which can increase the risk of fractures in adults and children. Your healthcare provider should check you for this during treatment with SOHONOS.
- new or worsening mental health problems that may include depression, anxiety, mood changes, and suicidal thoughts and behaviors. If you have a history of mental health problems, you may be at a higher risk of developing these side effects. Call your healthcare provider if you develop new or worsening mental health symptoms during treatment with SOHONOS. Your healthcare provider should monitor you for signs of depression and refer you for appropriate treatment, if necessary.
- vision problems (night blindness) which may cause difficulty seeing at night or in low lit areas. Your healthcare provider should send you to see an eye specialist if you experience vision problems.
The most common side effects of SOHONOS include:
| dry skin | rash | nausea |
| dry lips | skin peeling | muscle and joint pain |
| hair loss | drug eruption | dry eyes |
| itching | skin irritation | headache |
| redness | swelling and small cracks in corner of the mouth | fatigue |
| dry skin | swelling and small cracks in corner of the mouth |
| dry lips | nausea |
| hair loss | muscle and joint pain |
| itching | dry eyes |
| redness | headache |
| rash | fatigue |
| skin peeling | |
| drug eruption | |
| skin irritation |
These are not all the possible side effects of SOHONOS. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
WHAT IS SOHONOS?
SOHONOS is a prescription medicine used to reduce the amount of new heterotopic ossification in adults and children 8 years of age and older for females and 10 years and older for males with fibrodysplasia ossificans progressiva (FOP). SOHONOS is not recommended for females younger than 8 years of age or males younger than 10 years of age.
Please see full Prescribing Information, including Medication Guide with IMPORTANT WARNING.



